As previously reported, the Lenawee Health Department hosted an informal meeting on my request to discuss the health risks for citizens of Adrian and Adrian and Raisin Township imposed by the large flare (see below) on Witt Farm (Adrian-25). The meeting took place on Friday, February, the 21st.
The following people attended this meeting:
Patsy Bourgeois (Health Officer, Lenawee County Health Department), Martha Hall (Environmental Health Director, Lenawee County Health Department), Elise Garcia (Adrian Dominican Sisters), John Kuschell (Adrian resident, recycling activist), Jim Berryman (Mayor of the City of Adrian), Shane Horn (Interim Administrator of the City of Adrian), Sersena White (Senior Environmental Engineer, Michigan DEQ Air Quality Division), Terry Wright (Permit specialist, DEQ Air Quality Division), Kristie Shimko (Geologist, DEQ, Office of Oil, Gas, and Minerals, Lansing District Office, covering Jackson, Hillsdale and Lenawee Counties), Terry Collins (Lenawee County Commissioner for District 6: Westside of the City of Adrian and retired police chief of the City of Adrian), Amy Wassmer (Adrian resident and environmentalist), and Tom Wassmer (Assistant Professor of Biology at Siena Heights University and Environmentalist).
Q: What was the meeting about?
A: Health Risks of the Massive Flare Within City Limits
Q: What is a flare?
A: A flare is a way to get rid of “waste” gas in oil and gas operations. Instead of using perfectly useful and energy-rich natural gas, many oil and gas companies do not want to invest in the technology needed to capture and market the gas and rather burn it off. This is not only a waste but produces greenhouse gases warming up our planet unnecessarily, and also set free poisonous and cancerous by-products such as benzene (more below)

Q: Where is there a flare on Witt Farm ? – I do not see a flame!
A: The flare is enclosed (hidden) from the view in a empty oil container with the top removed. This is supposable done to avoid that people are reporting a fire, and to allow for a better burning rate (see below). However, I am not sure that it is (also) a psychological trick to avoid that people are realizing what happens and become aware what is done here to our health and the environment.

Location of the flare (pink rectangle)
But what is inside of the empty tank barrel is this: A massive flame that burns up to 770,000 cft of gas per day combining the maximum permitted amount of flare gases of currently 7 wells, which Savoy can further increase to a maximum of 10 wells - and then 1,100,000 cft of gas burned per day. In size and dimensions something like the open flare shown below:

If this flame burns at an efficiency of 96-100% then all is good. A minimal amount of methane, which is 100x as bad as a greenhouse gas as carbon dioxide and also minimal poisonous and cancerous hydrocarbons such as benzene are emitted. BUT, flares rarely burn at this efficiency due to winds and inconsistent flow rates of the "waste" gas itself. The consequence is a more orange flame with more soot (which you cannot see or cannot see very well in a enclosed flare) but also bad odor. If a flare smells then it is NOT burning at high efficiency. Some of this smells indicate that more methane escapes uncombusted, which makes the impact of a flare on global climate change worse. Much more concerning than bad odor are the higher concentrations of poisonous and cancerous hydrocarbons such as benzene that most people cannot smell at these concentrations but they can still cause short-term and long term health problems (see below).
Before being burned - the gas contains most probable (if Savoy is honest) the below components. In March 201, shortly after establishing the flare on Witt Farm (Adrian 1-25), Savoy Energy took a sample of the gas flared at Witt Farm (Adrian 1-25) and send it to a lab in Traverse City. I am puzzled that Savoy was not required to have the DEQ take the sample and send it to DEQ’s own environmental laboratory via a costly “Chain of Custody” to guarantee that it is the unhampered sample taken on location that arrives at the lab. This is the costly procedure every citizen has to go through to have air samples tested – why does the same rule not apply to a well-off company? However, the analysis still shows the presence of toxic and cancerous compounds such as BTEXs (benzene, toluene, ethylbenzene, and xylenes), most importantly benzene. Below are the analytical results for the sample(s) received by SPL on Thursday, March 28, 2013.
Q: The amount of benzene and other pollutants is surely too low to be concerned – otherwise the authorities would not allow flaring?
A: Unfortunately, local and regional authorities have no say in these matters and the DEQ and EPA are giving the oil and gas industry still unprecedented rights or have no legal tools to ask those industries to be more responsible. There is little information and research out there as these industries are very powerful and keep politicians, the court system, and even independent science out of their business. I already cited the exceptional study by James Argo in another blog post. Even in a well-burning flare, the following amounts of chemicals were released:
| On site characterization | Mg/m3 | Thermal Absorption > 10 mg/m3 | mg/m3 | Solvent Extraction > 10 mg/m3 | mg/m3 |
| Hydrogen | 20 | Pentane | 12.8 | Subst benzene | 9.83 |
| CO | 15.7 | 3-penten-1-yne | 19.3 | Azulene | 21.2 |
| CO2 | 4890 | Benzene | 144.5 | Subst benzene | 11.47 |
| Carbon | 54.2 | 1,5-hexadiyne | 48.2 | Naphthalene | 99.39 |
| Methane | 103.8 | Methyl benzene | 27.5 | 2-methyl naphthalene | 9.25 |
| Ethylene | 29 | Ethyl benzene | 13.7 | 1-methyl naphthalene | 6.18 |
| Acetylene | 53.7 | Ethynyl benzene | 94.8 | 1,1'-biphenyl | 58.7 |
| Ethane | 9.9 | Ethenyl benzene | 82.1 | Biphenylene | 42.81 |
| C3 HC's | 11.7 | Benzaldehyde | 18.7 | 1H phenalene | 21.01 |
| C4 HC's | 6.4 | Phenol | 26.4 | 9H fluorene | 41.09 |
| Benzene | 116.5 | Naphthalene | 88.7 | Phenanthrene | 10 |
| Toluene | 18.2 | 1,1'- biphenyl | 16.1 | Anthracene | 42.11 |
| Xylenes | 29.8 | Biphenylene | 19.1 | Fluoranthene | 51.35 |
| Styrene | 75.5 | Acenaphthalene | 23.2 | Pyrene | 32.37 |
| Ethynyl benzene | 79.6 | 4-methyl; pyrene | 9.1 | ||
| Naphthalene | 77.2 | 1 methyl pyrene | 8.4 | ||
| Other HC's | 128.5 | 38 Other HC's | 132.8 | Benzo(ghi)fluora nthene | 10.18 |
| CE | 65.0 % | Cyclopenta(cd)- pyrene | 29.77 | ||
| Benz(a)- anthracene | 17.33 | ||||
| 48 Other HC's | 94.47 |
I just want to focus on benzene (highlighted in red) as this is really bad stuff.
116.5 mg benzene/m3 when the gas is flowing at 6 m3/min in a sweet gas flare or 699 mg benzene released per minute. The authors find that the sweet gas flare has a maximum benzene concentration of 0.06 µg/m3 at about 500 m from the flare and measurable amounts at 5 km. These values are based on much smaller flares. The one on Witt Farm is permitted to flare up to 1,100,000 cft of gas per day – this would mean about 3.6x as much as the standard flare used in Argo’s calculations resulting in a benzene concentration of 0.22 µg/m3 at about 500 m and 0.09 µg/m3 at 5000 m. This is just below a lifetime risk of 1:1,000,000 for adult leukemia corresponding to an annual average benzene concentration of 0.096 ug/m3. Again this assumes 96-100% combustion rate (Values from Argo 2001, adjusted to likely concentrations and flow rates of the Witt Farm flare).
Combustion efficiency (CE) in a flare is severely affected by wind. Flares burning waste will only operate at
95+% if winds are less than about 2 kph. Winds of 25 kph will cut the CE to below 55-65%. In Adrian it is unlikely to have winds below 2 kph for at least 90% of the year! Enclosed flares are a little better but the frequency of bad odor from the flare confirms that it is most of the time NOT burning very well. Therefore the estimated concentration of benzene in the plume (wind direction) is most likely above the acceptable maximum value of 0.096 µg/m3 over at least 5 km from the source and an estimated peak value of benzene at about 500 m of about 4.8 µg/m3 or 50 X the acceptable maximum. This means: There might be an elevated risk for cancer may be present in the first 2.5 to 5 km from the flare. Residents in this zone have the potential to be affected by benzene exposure (described below). This concentration of benzene is below the odor threshold for most people (values and text passages from Argo 2001, values are representative for the Witt Farm as well).
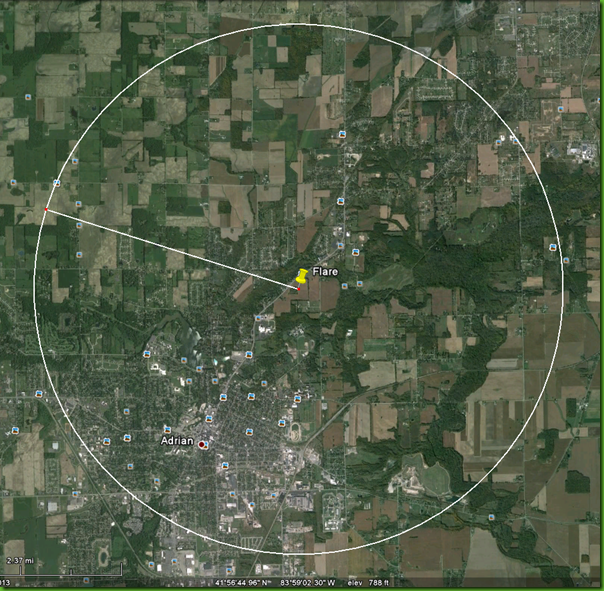
The flare on Witt Farm and the 5km radius around the flare in which to expect health impacts due to benzene alone. At least 15,000 people live within this circle.
Q: What are the health risks of benzene exposure?
A: Benzene is a systemic toxicant in humans at any concentration and a cause of aplastic anemia (deficient red blood cell production). The major effect of benzene in the body is depression of bone marrow leading to pancytopenia, (a general depression of erythrocytes (red blood cells), leukocytes (white blood cells) and thrombocytes (platelets)). A widespread reduction in erythrocytes in a population will lead to a general increase in morbidity (Argo 2001).
Benzene is a known human carcinogen, causing leukemia; it is non-mutagenic. An annual time weighted average concentration (TWA) for a risk of 1 in a million is an annual average concentration of 0.096 µg/m3.
The odor threshold (threshold is the concentration when an average person becomes aware of an odor) of benzene is 4.5 mg/m3 and the odor is described as sweet. An average person will become aware of the presence of benzene at a concentration 4500 / 0.096 = 46,800 x maximum acceptable value for annual exposure of a risk of 1:1,000,000. An average person can be at risk of leukemia and never be aware, take steps to protect or otherwise act in defense of their health and integrity (Argo 2001).
Increased morbidity and elevated risk of leukemia is everywhere possible for residents within 5 km under these conditions. Those most at risk of long-term health effects are persons under age 30 (Argo 2001).
Further Information can be found using the below literature:
- D'Andrea MA, Singh O, Reddy GK. 2013. Health consequences of involuntary exposure to benzene following a flaring incident at British Petroleum refinery in Texas City. American journal of disaster medicine 8(3):169-79. Abstract on PubMed
- D'Andrea MA, Reddy GK. 2014. Health Effects of Benzene Exposure among Children Following a Flaring Incident at the British Petroleum Refinery in Texas City. Pediatric hematology and oncology 31(1):1-10. Abstract on PubMed
- Argo J. 2001. Unhealthy Effects of Upstream Oil and Gas Flaring. Sydney, NS, Canada: SAVE OUR SEAS and SHORES (SOSS). Available from: http://www.sierraclub.ca/national/oil-and-gas-exploration/soss-oil-and-gas-flaring.pdf
Q: What can we do to make sure that we are not getting sick from this benzene exposure?
A: Please refer to my next post in which I will summarize the outcome of the meeting.






